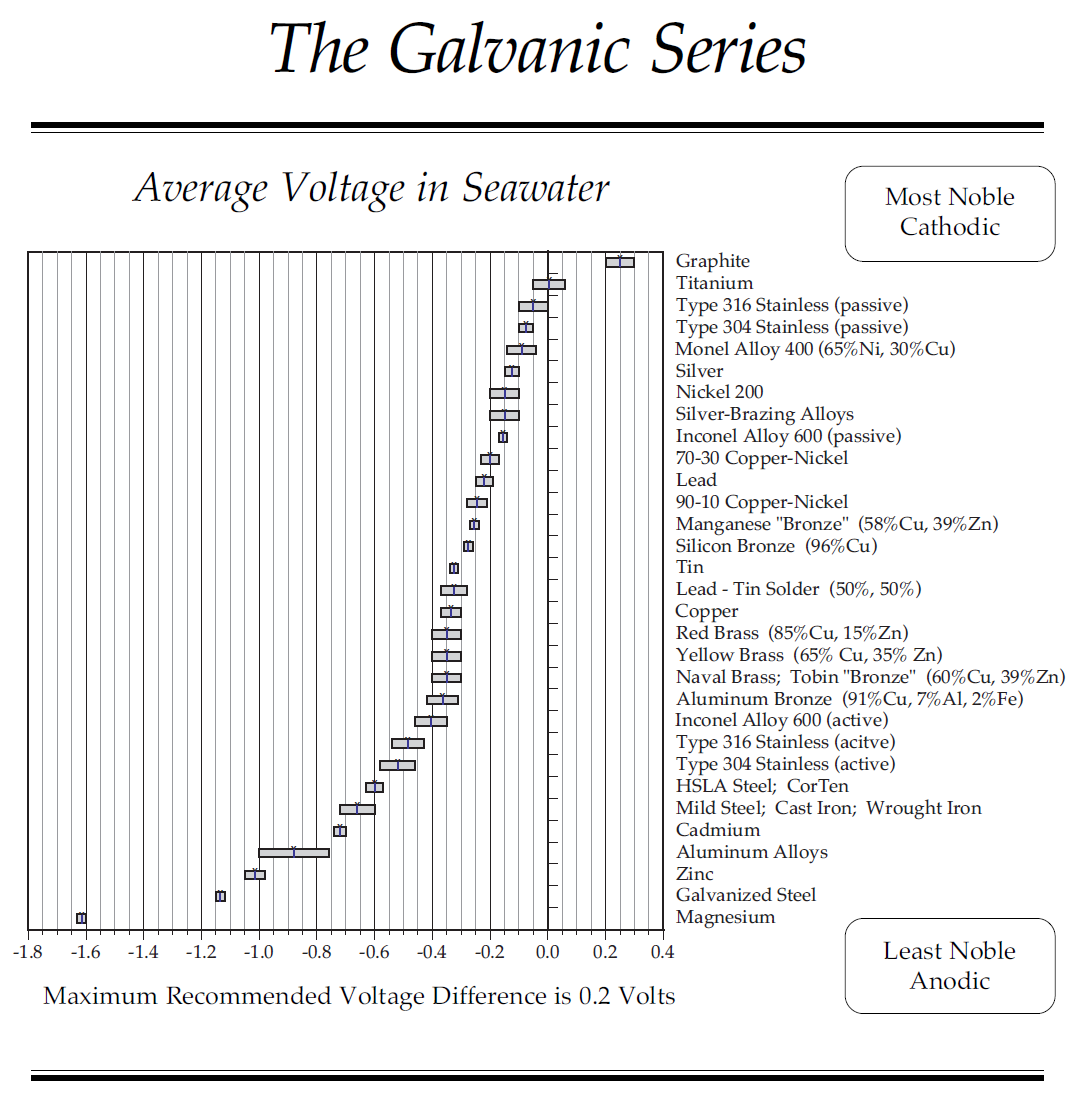
George-seems we are talking about two different things. First, whether copper prevents growth, it does. That is no secret, hence the copper in the paint. But, as far as I am aware, it is the direct contact with copper that prevents growth. Copper release copper ions that are antimicrobial and algeacidal and prevent attachment to hulls. Thus the need for direct contact. From your comment, it appears that the underwater metal parts are not connected in any way to the copper on the hull. Even if they were grounded to the copper plate, I can't see where that could possibly have any effect. If there were an effect, with a connection, it would seem it would almost have to be electrical. That is, the immersion of the copper plate in salt water is creating a small current that keeps bad things from attaching. that is the only explanation I can see.
The second issue is the corrosion issue. Again, here I think whether or not the metal parts are connected to the copper plate would be important. No secret that current flows to the lesser noble metal, in this case, the matel parts. That would seem to me to accelerate, not retard any corrosion issues with those parts. If there is no connection, again I cannot see how the copper plate could have any effect. It should be not different than simply not having zincs on the metal parts.
Not sure if any of this makes any sense, I am not an electrician or corrosion person, but it just seems logical to me.