A DMM can be used to check continuity of the zinc to the metal it is supposed to protect when the boat is out of the water. In fact the resistance should be checked. Failure to do that may result in a zinc just looking pretty yet doing nothing. If zincs have not degraded or minimally degraded then be suspicious.
Sometimes even what looks like a clean mounting surface is not as clean as you think. I DMM check all my zincs and sometime find that one of them, even though TIGHT on the shaft or the steel keel, is not actually in contact. I now take my grinder or Scotchbrite to the zinc surface, lightly, so it is bright, and if need be hit the mounting surface with a Scotchbrite pad, wire wheel or sanding disc as needed.
I check the prop to shaft resistance also although I've never had a problem there. Yet the possibility exists that even with a brush and shaft zincs that the connection will not allow them to work.
http://www.boatzincs.com/corrosion-reference-electrode-specs.html
will take you to a silver-silver chloride half cell that is used in conjunction with a meter to take voltage readings of the metals immersed in seawater.
There are voltage levels for bronze, SS, Alum., and steel and the reading will give you a good idea of how they and the zincs are doing.
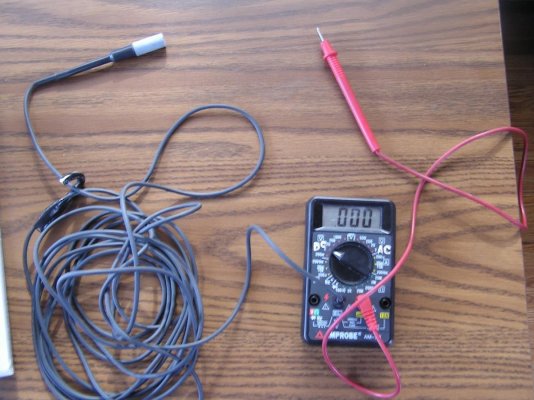
You will need your own DMM, and a long ground lead that is used to touch the metals to get the reading. The CELL goes into the seawater.
I bought the meter kit years ago. I didn't know about Boatzincs at the time so spent a lot more money that I would have to now.
So assuming that most of us have a DMM and can cobble a 20ft probe lead this is one way to go.
I check my voltage levels at least twice a year. A couple of times I found a piece of metal that was not being protected so was able to take action rather than waiting to find out at haulout.
The setup can also be used to check for AC leakage. By disconnecting shore power and testing, then replugging shore power, if all is well than there should be NO change. If there is you may have a problem. You can also check to see if any AC ripple shows with a DMM which is another way.
I would get those props checked. Diver. But also ensure there is zincing that is actively protecting the props. They may be good with just a light pinking and if dealt with properly now could save you a bunch of money down the road.